Client
SK Biotech
Location
Ireland
Services
- Process Engineering Services
Sectors
- Life Sciences
Sub-sector
- Pharma & Biotech
Period
01/05/2022
Callaghan | RED (CRE) executed a Basis of Design Engineering Study (BOD) for a new large scale Active Pharmaceutical Ingredients (API) Manufacturing Plant at SK biotek’s existing manufacturing campus located in Watery Ln, Townparks, Swords, Co. Dublin, Ireland.
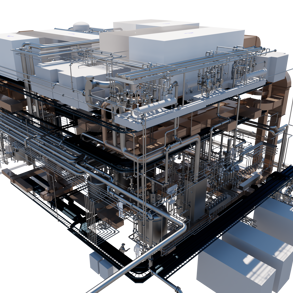
The proposed new P2 process building will be constructed within the existing P2/P5 building footprint, incorporating a 3-story steel frame and metal clad structure spanning over 1024 m2. It will serve as a dedicated space for chemical synthesis, API intermediate isolation, and finished API product manufacturing. The expansion aims to increase output for existing customer API products and explore new product lines. The development process involved collaboration between SK biotek stakeholders and Callaghan | RED designers, resulting in a Basis of Design (BOD) report that defined project requirements and emphasized cost control.
The report included assessments such as P&IDs, Hazop studies, and compliance with active ingredients containment, explosion prevention, and GMP guidelines. A comprehensive 3D model of the development was created, enabling identification of spatial constraints, equipment layouts, and coordination requirements. The BOD report confirmed technical feasibility, alignment with SK biotek engineering guidelines, and provided an overall project budget estimate within +/- 20% accuracy. Commercial information and technical IP remain confidential. The BOD report supports the SKB biotek stage gate process and sets the foundation for future detailed design engineering.
TALK ABOUT THIS PROJECT
Unlock the potential of your engineering projects with our expert consultation services. Our team of knowledgeable professionals is ready to provide guidance and innovative solutions tailored to your specific needs. Contact us today to discuss how we can help you achieve your goals.
Get in touchPROJECT SUMMARY
Basis of Design Engineering Study (BOD) for a new large scale Active Pharmaceutical Ingredients (API) Manufacturing Plant. The purpose of the BOD was to ensure that the SK biotek requirements for the various elements of the project were clearly defined to a Basis of Design level of detail with emphasis on cost control to facilitate a financial appraisal of the investment which was identified to be cost sensitive from the onset of the project. The BOD conclusion was that the project is technically feasible and in alignment with SK biotek engineering design guidance requirements. The BOD report supported the SKB biotek stage gate process by defining key elements of the design and including an overall +/- 20 % project budget estimate.
4 MONTHS
Full engineering Basis of Design and Capital Cost Estimate completed in 4 months.
P&IDs
Full set of P&IDs developed with limited information of the processes that will be transferred and allowing for multipurpose operation and maximum flexibility in the manufacture of API ingredients.
Damage Limitation Design
Explosion Protection and Damaged Limitation Construction Design.
Callaghan|RED completed a technical basis of design in a very tight timeframe for execution and taking into consideration our budgetary constraints.Barry Welsh
Process Engineer, Sk biotek